Mechanisms of Growth (Hypothesis) (For Professionals)
Mechanisms of Growth (Hypothesis) (For Professionals)
Two schools of thought exist, one states that SK develops from an accumulation of senescent cells, whereas the other states that cells hyperproliferate. Ki-67 is a marker of proliferation that links itself to the Dense Fibrillary Component of the nucleolus (reference). We used ki-67 marking of SK to try to find our own answer.
Hyperproliferation theory?
The proliferation hypothesis of keratinocytes in SK has been demonstration by different means. The first experience shows that SK could be due to a benign epidermal hyperplasia. The HUMARA gene contains two alleles located on the X chromosome. Normally in a normal skin specimen, both alleles are expressed quite equally. Indeed, in a clone of cells, only one of the alleles is expressed, because the same X chromosome is inactivated by lyonisation{Allen, 1992}, in a clone, whereas in normal {Nakamura, 2001} skin (polyclonal), the inactivated HUMARA gene allele isn’t always on the same X chromosome.
SK according to Hallermann{Hallermann, 2004} et al. seem to have a extra chromosome. They curetted 4 SKs and analysed by genomic hybridization. No chromosome was found contrary to an extra chromosome 5 in the basal cell carcinoma. This study suggests that no chromosomal anomalies are present but the aneuploidy is the result of a reactive process. But genomic hybridazation cannot detect chromosomial aberrations of less than 2 kilobases, the authors thus acknowledge that a small chromosomal change can be present.
According to Nakamura et al. {Nakamura, 2001}, there is inactivation of one of the two genes of the Human activated receptor of androgens (HUMARA). This gene is located on the X chromosome and one of the alleles is thus inactivated. The quantitative detection of the HUMARA gene was done by polymerase chain reaction on 38 non genital specimens in women. The specimens were compared with 5 specimens of normal skin. After digestion with a restriction enzyme (HhaI), one of the two spikes disappeared in 20 of 33 SK specimens. ON normal skin, the two spikes were present. As a conclusion 25 of 33 specimens of SK could be considered as monoclonal. As a conclusion SK without being without being necessarily cancerous appears to behave in a hyperproliferative fashion.
A clue to suggesting a possible neoplasic nature of SK keratinocytes is suggested by their cytosqueletton type expression{Nindl, 1992}. High molecular weight cytokeratin (1/68kD, 19/56,6 kD) was replaced by simple cytokeratin (8/52kD, 18/45kD) which is found in neoplasic cells. Let us underline nevertheless that such cytokeratin expression is found in embryonic cell lines and that the study only concerned 10 specimens.
Survivin is an inhibitor of apoptosis found in many neoplasms {Velculescu, 1999}. Bowen et al (2004) {Bowen, 2004} measured the expression of survivin (immunohistochemistry and in situ hybridation) in different epithelial neoplasms, based on the fact that bcl-2 – another inhibitor of apoptosis – is slightly elevated in SK{Nakagawa, 1994} . Survivin was found in all SKs. Bowen et al. {Bowen, 2004} detected keratinocyte proliferation by PCNA (proliferating cell nuclear antigen) marking, 13,9% of keratinocytes were stained, compared with 6,4% in normal skin. The TUNEL method was also used by Bowen et al.{Bowen, 2004} to detect apoptosis in fixed tissues, but no statistically significant results between SKs and normal skin samples were shown. Therefore, the authors concluded to a probable hyperplasic status in benign or malignant neoplasic cells. A rententional model cannot be excluded, even if survivin expression was shown to be present. Indeed apoptosis is inhibited in senescent cells. No inhibition of apoptosis following apoptotic stimuli on cell cultures was done, as Nakamura et al did (2003){Nakamura, 2003}.
Proliferation of SK was evaluated by histological means. Ki-67 is a proliferation marker protein consisting of a doublet of 345kD and 395kD which associates itself to the dense fibrillary component (DFC) of the nucleolus {Verheijen, 1989}. Its function is unknown but it shows similarities with proteins like forkhead-associated domain (FHA) in the amino terminal region of the Ki67 protein, sequences that resemble FHA are located in proteins (DUN 1 protein kinase for example)involved in the regulation of the cell cycle{Hofmann, 1995}.
A hyperproliferative state is suggested by the presence of a strong immunoreactivity of the antibody Mab263 which marks the growth hormone receptor{Ginarte, 2001}. Other benign and malignant cutaneous proliferative entities where the growth hormone receptor was present included histiocytomas, melanomas and acrochordons. An interesting finding is that immunoreactivity is weaker in malignant cutaneous tumours (squamous cell carcinoma and basal cell carcinoma). Moreover, the intensity was inversely proportional to cellular differentiation. As a conclusion, proliferative cutaneous entities such as SK express growth hormone receptors, but malignant ones seem less disposed to do so.
The stimulation of the epidermal growth factor receptor (EGF-R) induces proliferation. And that receptor has been found increased in SK. {Nanney, 1992} Stimulation of epidermal growth factors induces cell proliferation. In SK only in vivo proliferating lesions showed an elevated immunostaining or EGF-R and 125I-EGF binding sites. Conversely, non clinically proliferating SKs showed a normal pattern of immunostaining. Comparatively, viral lesions such as verruca vulgaris show no EGF-R immunostaining (see viral etiology).
{Gushi, 2003}EGF-R expression seems to correlate with the clinical malignity of an epithelial tumour {Gushi, 2003}. In benign ones such as SK, there is an ordered expression of EGF-R whereas in squamous cell carcinoma, there is an irregular distribution, EGF-R being also accumulating in the cytoplasm.
SK of irritated type displays less EGF-R {Nanney, 1992} expression and could represent an involution phase of SK {Pesce, 2000}(see retentional theory).As a summary, SK seems to show quiescent and proliferative states with altered EGF-R receptor expression. The ordered distribution of EGF-R suggests that SK, if a neoplasm, is benign.
As will be mentioned later p16 in the retentional model also seems to be found in hyperproliferative states. P16 is present in spinous cell carcinoma and its premalignant skin lesions. Although it is NOT overexpressed in SK according to the study by Salama et al (2003){Salama, 2003}, it is worth mentioning, because it has been found present in the studies by Nakamura et al (2003){Nakamura, 2003} (retentional model).
As explained in the retentional model, p16 is a “tumour suppressor protein”, which inhibits Rb phosphorylation (thus inactivation) by cyclin dependant kinases 4 and 6 {zur Hausen, 1996; Koh, 1995; Sherr, 1996}, thus arresting the cell cycle in G1.
Mutations of p16, p53 or those increasing the activity of CDK 4 or 6 exist {Soufir, 1999}. These mutations induce a precocious passage in the S phase while being very briefly arrested in G1. Indeed Rb is inactivated by phosphorylation thus liberates E2F, thus premitting the cell cycle to progress in S1. Inactivated Rb is absent where p16 is present. Conversely, p16 is absent when the Rb gene is intact {Kratzke, 1995}.
Salama et al (2003) {Salama, 2003} detected p16ink4a by “immunohistochemistry” and large core microarray analysis done on 90 of 107 biopsies of Bowen’s disease, but 4 only of actinic keratosis, and none of 10 SKs. P16ink4a is also present in squamous cell carcinomas {Klaes, 2001}.
The increase in expression of p16 with or without a modification of its promoter region could be in reaction to a neoplasic phenomenon, to arrest the cell cycle although inefficiently, to allow cell repair. As mentioned by Pavey et al (1999){Pavey, 1999} for SKs, the increase in expression of p16ink4a is due to UV radiation, although p16 seems inactive in squamous cell carcinomas{Kubo, 1997}.
The following is not specific to seborrheic keratosis but represents an attempt to link two histologically close lesions: SK and Basal Cell Carcinomas. Basal cell carcinomas are not true cancers. They seem to overproliferate in response to an activation of the hedgehog pathway. Even if this pathway has not been put into evidence in seborrheic keratosis, it could offer some interesting therapeutical opportunities. Basal cell carcinomas and SK both stem from the follicular apparatus.
The sonic hedgehog pathway (see figure above) {Matsumura, 2002} which has been studied in the drosophila, the linkage of the hedgehog protein (Hh) to the patched receptor protein (PTC) {Stone, 1996; Marigo, 1996} uninhibits (by an unknown mechanism) the smoothened protein (SMO), which is normally inhibited by PTC {Alcedo, 1996 }. The SMO signal pathway induces Cubitus interuptis (Ci) to dissociate from the Costal 2-Fused-Su (COS2-Fu-Su) complex and after migrating to the nucleus to switch transcription target genes such as Wingless (WG){Von Ohlen, 1997} (Von Ohlen and 1999) {Von Ohlen, 1997}, Decapentapleic (DPP) and PTC (PTCH 1 and 2). Fu is a serine threonin kinase {Alves, 1998}. COS2 links the complex to microtubules. To summarize, Hh induces growth and differentiation.
As already mentioned, the sonic hedgehog pathway has interesting therapeutic options. Two substances have a known inhibitory action on the sonic hedgehog pathway. The first one is cyclopamine, which as demonstrated by Berman and Thayer was found to inhibit the growth of medulloblastomas. Cyclopamine, an alcaloid steroid acts by linking the heptahelical bundle of SMO, although the inhibitory mechanism is unknown. Cyclopamine as its name suggests is teratogenic, which produces cyclopia when administered to sheep. The second substance is megalin (gp330/LRP-2), an intracellular receptor which is expressed in embryonic epithelia and which has a role in growth {McCarthy, 2003}. The megalin receptor acts by linking Hh protein and retinol. Hh action is thus inhibited.
Unfortunately, a role of the sonic hedgehog pathway in SK is not proven and the two substances mentioned are not in the present acceptable therapeutic options. SK is not present in SK as it is in other malignant neoplasms: small cell lung carcinoma, medulloblastoma, basal cell carcinoma , suggesting it could be a mere benign hyperproliferative state.
Accumulation by senescence theory?
Ki67 has been measured in SK {Matsuta, 1996} by immunohistochemistry and the growth fraction (number of Ki 67 positive cells divided by the total amount of cells) amounted to 9.7% +/- 3,1%. The value was 3 times lower than in basal cell carcinoma (32.9 +/- 10,5%). Even if we take into account the lower growth fraction of ki-67 positive cells which is normally defined, there are laboratory variations. Moreover over studies{Gilhar, 2004 #429}that normal skin in old age is around 12 %. If we try to compare the two groups, SK appears to be hypoproliferating.
Nakamura and al think that SK is provoked by an accumulation of senescent keratinocytes expressing p16ink4a and unable to enter apoptosis. They(2003){Nakamura, 2003} found that all 10 tissue samples of acanthotic SKs expressed P16 (immunohistochemistry) in the whole epidermal thickness, p16ink4a being only present in the granular layer in the normal skin epithelium. Conversely, the granular layer is the layer of senescent cells in the normal epidermis. The following paragraph is summarised by the figure below.
CDKN2A is the gene which codes for protein 16 (p16) ink 4a, which is an inhibitor of a cyclin dependant kinase (CDK) {Castellano, 1997}. p16ink4a links CDK4 or 6, which are rendered unable to phosphorylate Rb, thus the cell cycle is blocked in the G1-S phase transition {Guan, 1994; Lukas, 1995; Koh, 1995}. This mechanism is probably responsible of senescence {Alcorta, 1996; Stein, 1999; Kiyono, 1998}.
Nakamura et al (2003) {Nakamura, 2003} also found an increased cell survival time, perhaps due to an inhibition of entry into apoptosis. No DNA fragmentation (TUNEL method) was found in 4 of 5 specimens. Thus keratinocyte cells cannot disappear as corneocytes. Indeed, p16ink4a in addition to stopping the cell cycle is known to inhibit apoptosis by the p19-p53 pathway {Quelle, 1995; Wu, 1994; Nevins, 1992; Qin, 1994} also found an increased cell survival time, perhaps due to an inhibition of entry into apoptosis. No DNA fragmentation (TUNEL method) was found in 4 of 5 specimens. Thus keratinocyte cells cannot disappear as corneocytes. Indeed, p16 in addition to stopping the cell cycle is known to inhibit apoptosis by the p19-p53 pathway {Quelle, 1995, Nakamura, 2003} also found an increased cell survival time, perhaps due to an inhibition of entry into apoptosis. No DNA fragmentation (TUNEL method) was found in 4 of 5 specimens. Thus keratinocyte cells cannot disappear as corneocytes. Indeed, p16ink4a in addition to stopping the cell cycle is known to inhibit apoptosis by the p19-p53 pathway {Quelle, 1995; Wu, 1994; Nevins, 1992; Qin, 1994}. No p53 was found, which according to Soini et al. {Soini, 1994} suggests that the SK samples were non proliferative.
Authors’ experience
We tried to see if SK was due to a hyperproliferation or an accumulation of cellular material.
Methods: We took after approval of the ethics committee 36 seborrheic keratosis and 15 specimens of control skin (plastic surgery, inflammatory skin (biopsies of suggested lymphomas with ki.67 analysis but with no epidermotropism or atypical cells) which were sent for histological analysis and ki-67 marking (method in detail). Two groups within the SK group were done: all histologies compatible with a reticulate SK or acanthotic SK were put in the RA (reticuloacanthotic group) and the hyperkeratotic SK were put in the AHCS group. There were no clonal specimens. We did not take into account the pigmentation of cells, nor their degree of irritation. The keratinocytes in SK and control skin was then done. All ki-67* fully positive cells and a fine estimate of the number of cells was done under a microscope lens of 40x magnification. An average of four adjacent fields was done. Then a ki-67 proportion index for control skin and SK was done by dividing the total number of cells.
*Only fully stained cells were counted, the other basal cells could have accumulated melanin which also appears brown.
“8” specimens of control skin and 5 specimens of seborrheic keratosis were stained by the TUNEL method. One specimen of control skin has irradiated by (ordi) UV B and then stained after 24 hours of immersion in a culture medium.
Results: The proportion of Ki-67 positive cells out of all SKS altogether equalled 2.3% +/- 0.3% whereas the one of control skin was 8.27% +/- 0.7%. One of the specimens was subtracted from the study because the proportion of ki-67 positive cells equalled 72%!There was no statistical difference between the two chosen histological types of SK( reticulo-acanthotic 2.0 +/- 0.5% versus acanthohyperkeratotic 2.6% +/- 0.3%. The difference was statistically significant between SK and the control group. The control group divided into two groups, one on inflamed skin and the other on normal skin showed a statistical difference with the inflammatory group showing a bigger percentage of ki-67 positive cells.
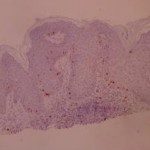
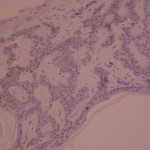
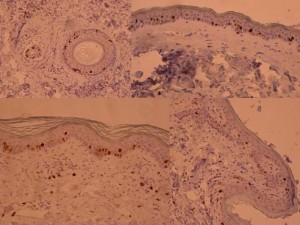
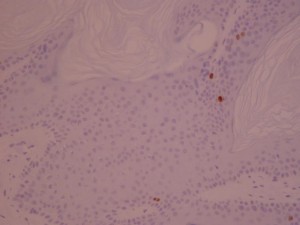
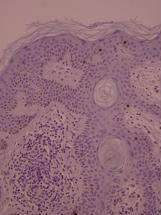
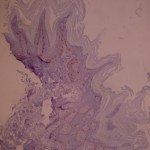
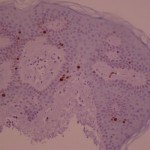 ki-67 staining of reticulo-acanthotic type of SKs.
ki-67 staining of reticulo-acanthotic type of SKs.
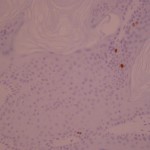
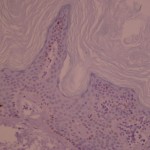
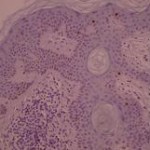
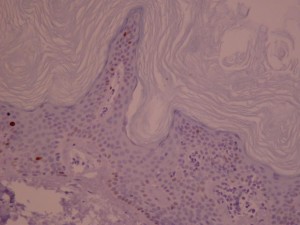
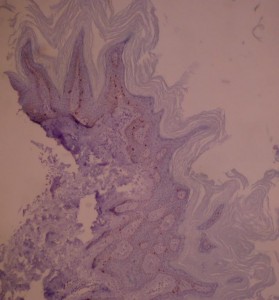
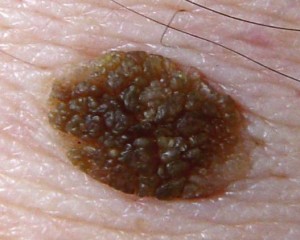
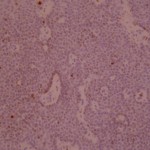
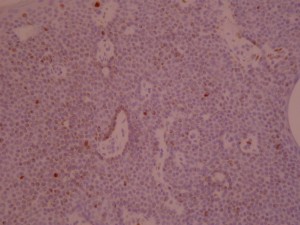
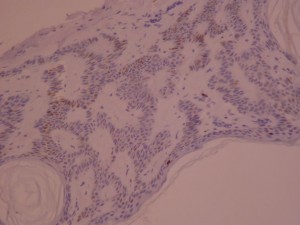
 Ki-67 staining of an acantho-hyperkeratotic type of SK. On the last picture, most of the basal staining comes from the accumulation of pigment as the clinical image suggests.
Ki-67 staining of an acantho-hyperkeratotic type of SK. On the last picture, most of the basal staining comes from the accumulation of pigment as the clinical image suggests.
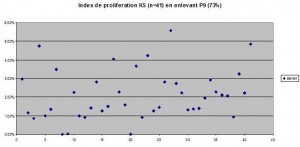
Proportion of ki-67 positive cells. The values equal to 0% (All Acanthohyperkeratotic) were taken into account. Even if these values were not taken into account, the difference between normal skin and acanthohyperkeratotic and SK as a whole would not be significantly changed, as the standard deviation would be decreased.
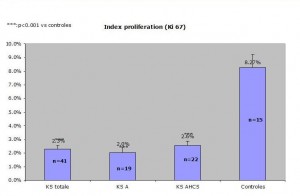
Comparaison of reticulo-acanthotic subtype of SK, Acanthohyperkeratotic, these two histological subtypes combined and normal skin. The average normal proliferating skin has an average of ki-67 positive cells of 8.27 percent which is at least 250% of the SK ki-67 percentage of positive cells (2.3%) KStotale=all SKs studied KS A=acanthotic or reticulate SKs KS AHCS=acanthotic and hyperkeratotic SKs Control=control group
Discussion: These results clearly show that the number of ki-67 in SK is either equal or lower than that of normal skin. The SK cells appear to be of a lower mitotic rate than control skin. Let us admit that we didn’t measure the basal layer proliferating index. But we can nevertheless say that if the number of cells increased because of a hyperproliferation, the number of ki-67 absolute number of cells would remain constant and that the proportion would remain unchanged.
There is no difference between the “proliferating” index between RA and AHCS SK. This means that although these lesions have clinically specific presentations (see our earlier experiment), there is no way of predicting the evolution of a lesion, a small and smooth SK can enlarge or remain static whereas an already large hyperkeratotic lesion can continue to grow.
The ki-67 marking of cells tends to be underestimated in our specimens. This is explained by a rigorous count of fully postitive ki-67 positive cells. The not fully stained cells resemble pigment accumulated in cells and thus the light accumulation of ki-67 positive cells were not counted. Thus the lower number of ki-67 postitive cells could be explained by laboratory methods or a lower amount of counted ki-67-positive cells( nomrmal values 9.7%+/- 3.1%{Matsuta, 1996}.
The difference in median age between the SK group and the control skin group could explain a bigger difference between SK in normal and control skin. According to {Gilhar, 2004}), group the ki-67 proliferating index is higher in a younger age group (%) than an older age group (12%). Even though the older age group shows a proliferating index of –%, laboratory differences make the comparaison between our group and theirs not comparable.
The TUNEL method of –SKs didn’t reveal any interDNA fragment staining in any of the SK and control skin specimens. The irradiated control skin (positive control) showed important DNA staining (photos à mettre)
Conclusion
As a conclusion two schools of thought exist, one advocating a retention of keratinocytes and the other a hyperproliferative state. A study in our department measuring the ki-67 index of “proliferation” of cells states that SK is the result of an accumulation of cells. The TUNEL experiment not showing any staining in the specimens of SK shows a supplementary argument to the presence of senescent cells, that is an inhibition of apoptosis.
One study relating bcl-2 expression is not incompatible with neither of the two schools of thought. Bcl-2 is a protein that inhibits apoptosis . Nakagawa et al (1994) {Nakagawa, 1994}evaluated bcl-2 expression in epidermal keratinocytic diseases. Immunohisochemical expression of bcl-2 in benign epithelial neoplasms (psoriasis, SK…) was compared with that of malignant skin tumours (squamous cell carcinoma, basal cell carcinoma…) Bcl-2 was totally absent in normal skin, scarcely present in SK, and present in all patients with squamous cell carcinoma. Malignant tumours thus have an enhanced cell survival. On the contrary, SK doesn’t behave like malignant neoplasms, and would be merely a bunch of keratinocytes in a hyperproliferative state.
As a conclusion inhibitors of apoptosis, whether survivin or bcl-2 are expressed in SK. Thus the retentionnal model is the plausible one our experience tends to support.
It would be interesting to measure by western blotting cell cycle proteins, ie. To repeat the Nakamura et al. of p16 expression. Another interesting path would be to measure SK keratinocytes’ resistance to apoptosis.
Pigmentation in SK: a mechanism explained by parallelism?
The mechanism in classical SK of pigmentation is unknown. It has nevertheless been studied in melanoacanthoma, a histologically close lesion to SK, which is sometimes considered as a subtype of SK, but is located in the muquous membranes. Pigmentation in SK is postulated to come from the activation of the endothelin receptor by endothelin-1 (ET-1) {Teraki, 1996}. ET-1, whose production is stimulated by UVB light {Kadono, 2001} is produced by endothelin converting enzyme 1 (ECE-1). Identification of ET-1 was done using immunohistochemistry and reverse transcriptase-polymerase chain reaction(RT-PCR) on seven acanthotic and pigmented SKs. RT-PCR revealed an increase in mRNA of ET-1 and tyrosinase, suggesting an ET-1 role in pigmentation. The postulted mechanism of action is as follows :.
Endothelin then acts on the neighbouring melanocytes (paracrine regulation) which express the endothelin receptor and induces melanocyte mitosis and production of melanin {Imokawa, 1992; Yada, 1991}.
Then the melanocytes transfer their melanosomes to the neighbouring keratinocytes {Sanderson, 1968}.
This advice is for informational purposes only and does not replace therapeutic judgement done by a skin doctor.
Contributors:
Dr Christophe HSU – dermatologist. Geneva, Switzerland
Two schools of thought exist, one states that SK develops from an accumulation of senescent cells, whereas the other states that cells hyperproliferate. Ki-67 is a marker of proliferation that links itself to the Dense Fibrillary Component of the nucleolus (reference). We used ki-67 marking of SK to try to find our own answer.
Hyperproliferation theory?
The proliferation hypothesis of keratinocytes in SK has been demonstration by different means. The first experience shows that SK could be due to a benign epidermal hyperplasia. The HUMARA gene contains two alleles located on the X chromosome. Normally in a normal skin specimen, both alleles are expressed quite equally. Indeed, in a clone of cells, only one of the alleles is expressed, because the same X chromosome is inactivated by lyonisation{Allen, 1992}, in a clone, whereas in normal {Nakamura, 2001} skin (polyclonal), the inactivated HUMARA gene allele isn’t always on the same X chromosome.
SK according to Hallermann{Hallermann, 2004} et al. seem to have a extra chromosome. They curetted 4 SKs and analysed by genomic hybridization. No chromosome was found contrary to an extra chromosome 5 in the basal cell carcinoma. This study suggests that no chromosomal anomalies are present but the aneuploidy is the result of a reactive process. But genomic hybridazation cannot detect chromosomial aberrations of less than 2 kilobases, the authors thus acknowledge that a small chromosomal change can be present.
According to Nakamura et al. {Nakamura, 2001}, there is inactivation of one of the two genes of the Human activated receptor of androgens (HUMARA). This gene is located on the X chromosome and one of the alleles is thus inactivated. The quantitative detection of the HUMARA gene was done by polymerase chain reaction on 38 non genital specimens in women. The specimens were compared with 5 specimens of normal skin. After digestion with a restriction enzyme (HhaI), one of the two spikes disappeared in 20 of 33 SK specimens. ON normal skin, the two spikes were present. As a conclusion 25 of 33 specimens of SK could be considered as monoclonal. As a conclusion SK without being without being necessarily cancerous appears to behave in a hyperproliferative fashion.
A clue to suggesting a possible neoplasic nature of SK keratinocytes is suggested by their cytosqueletton type expression{Nindl, 1992}. High molecular weight cytokeratin (1/68kD, 19/56,6 kD) was replaced by simple cytokeratin (8/52kD, 18/45kD) which is found in neoplasic cells. Let us underline nevertheless that such cytokeratin expression is found in embryonic cell lines and that the study only concerned 10 specimens.
Survivin is an inhibitor of apoptosis found in many neoplasms {Velculescu, 1999}. Bowen et al (2004) {Bowen, 2004} measured the expression of survivin (immunohistochemistry and in situ hybridation) in different epithelial neoplasms, based on the fact that bcl-2 – another inhibitor of apoptosis – is slightly elevated in SK{Nakagawa, 1994} . Survivin was found in all SKs. Bowen et al. {Bowen, 2004} detected keratinocyte proliferation by PCNA (proliferating cell nuclear antigen) marking, 13,9% of keratinocytes were stained, compared with 6,4% in normal skin. The TUNEL method was also used by Bowen et al.{Bowen, 2004} to detect apoptosis in fixed tissues, but no statistically significant results between SKs and normal skin samples were shown. Therefore, the authors concluded to a probable hyperplasic status in benign or malignant neoplasic cells. A rententional model cannot be excluded, even if survivin expression was shown to be present. Indeed apoptosis is inhibited in senescent cells. No inhibition of apoptosis following apoptotic stimuli on cell cultures was done, as Nakamura et al did (2003){Nakamura, 2003}.
Proliferation of SK was evaluated by histological means. Ki-67 is a proliferation marker protein consisting of a doublet of 345kD and 395kD which associates itself to the dense fibrillary component (DFC) of the nucleolus {Verheijen, 1989}. Its function is unknown but it shows similarities with proteins like forkhead-associated domain (FHA) in the amino terminal region of the Ki67 protein, sequences that resemble FHA are located in proteins (DUN 1 protein kinase for example)involved in the regulation of the cell cycle{Hofmann, 1995}.
A hyperproliferative state is suggested by the presence of a strong immunoreactivity of the antibody Mab263 which marks the growth hormone receptor{Ginarte, 2001}. Other benign and malignant cutaneous proliferative entities where the growth hormone receptor was present included histiocytomas, melanomas and acrochordons. An interesting finding is that immunoreactivity is weaker in malignant cutaneous tumours (squamous cell carcinoma and basal cell carcinoma). Moreover, the intensity was inversely proportional to cellular differentiation. As a conclusion, proliferative cutaneous entities such as SK express growth hormone receptors, but malignant ones seem less disposed to do so.
The stimulation of the epidermal growth factor receptor (EGF-R) induces proliferation. And that receptor has been found increased in SK. {Nanney, 1992} Stimulation of epidermal growth factors induces cell proliferation. In SK only in vivo proliferating lesions showed an elevated immunostaining or EGF-R and 125I-EGF binding sites. Conversely, non clinically proliferating SKs showed a normal pattern of immunostaining. Comparatively, viral lesions such as verruca vulgaris show no EGF-R immunostaining (see viral etiology).
{Gushi, 2003}EGF-R expression seems to correlate with the clinical malignity of an epithelial tumour {Gushi, 2003}. In benign ones such as SK, there is an ordered expression of EGF-R whereas in squamous cell carcinoma, there is an irregular distribution, EGF-R being also accumulating in the cytoplasm.
SK of irritated type displays less EGF-R {Nanney, 1992} expression and could represent an involution phase of SK {Pesce, 2000}(see retentional theory).As a summary, SK seems to show quiescent and proliferative states with altered EGF-R receptor expression. The ordered distribution of EGF-R suggests that SK, if a neoplasm, is benign.
As will be mentioned later p16 in the retentional model also seems to be found in hyperproliferative states. P16 is present in spinous cell carcinoma and its premalignant skin lesions. Although it is NOT overexpressed in SK according to the study by Salama et al (2003){Salama, 2003}, it is worth mentioning, because it has been found present in the studies by Nakamura et al (2003){Nakamura, 2003} (retentional model).
As explained in the retentional model, p16 is a “tumour suppressor protein”, which inhibits Rb phosphorylation (thus inactivation) by cyclin dependant kinases 4 and 6 {zur Hausen, 1996; Koh, 1995; Sherr, 1996}, thus arresting the cell cycle in G1.
Mutations of p16, p53 or those increasing the activity of CDK 4 or 6 exist {Soufir, 1999}. These mutations induce a precocious passage in the S phase while being very briefly arrested in G1. Indeed Rb is inactivated by phosphorylation thus liberates E2F, thus premitting the cell cycle to progress in S1. Inactivated Rb is absent where p16 is present. Conversely, p16 is absent when the Rb gene is intact {Kratzke, 1995}.
Salama et al (2003) {Salama, 2003} detected p16ink4a by “immunohistochemistry” and large core microarray analysis done on 90 of 107 biopsies of Bowen’s disease, but 4 only of actinic keratosis, and none of 10 SKs. P16ink4a is also present in squamous cell carcinomas {Klaes, 2001}.
The increase in expression of p16 with or without a modification of its promoter region could be in reaction to a neoplasic phenomenon, to arrest the cell cycle although inefficiently, to allow cell repair. As mentioned by Pavey et al (1999){Pavey, 1999} for SKs, the increase in expression of p16ink4a is due to UV radiation, although p16 seems inactive in squamous cell carcinomas{Kubo, 1997}.
The following is not specific to seborrheic keratosis but represents an attempt to link two histologically close lesions: SK and Basal Cell Carcinomas. Basal cell carcinomas are not true cancers. They seem to overproliferate in response to an activation of the hedgehog pathway. Even if this pathway has not been put into evidence in seborrheic keratosis, it could offer some interesting therapeutical opportunities. Basal cell carcinomas and SK both stem from the follicular apparatus.
The sonic hedgehog pathway (see figure above) {Matsumura, 2002} which has been studied in the drosophila, the linkage of the hedgehog protein (Hh) to the patched receptor protein (PTC) {Stone, 1996; Marigo, 1996} uninhibits (by an unknown mechanism) the smoothened protein (SMO), which is normally inhibited by PTC {Alcedo, 1996 }. The SMO signal pathway induces Cubitus interuptis (Ci) to dissociate from the Costal 2-Fused-Su (COS2-Fu-Su) complex and after migrating to the nucleus to switch transcription target genes such as Wingless (WG){Von Ohlen, 1997} (Von Ohlen and 1999) {Von Ohlen, 1997}, Decapentapleic (DPP) and PTC (PTCH 1 and 2). Fu is a serine threonin kinase {Alves, 1998}. COS2 links the complex to microtubules. To summarize, Hh induces growth and differentiation.
As already mentioned, the sonic hedgehog pathway has interesting therapeutic options. Two substances have a known inhibitory action on the sonic hedgehog pathway. The first one is cyclopamine, which as demonstrated by Berman and Thayer was found to inhibit the growth of medulloblastomas. Cyclopamine, an alcaloid steroid acts by linking the heptahelical bundle of SMO, although the inhibitory mechanism is unknown. Cyclopamine as its name suggests is teratogenic, which produces cyclopia when administered to sheep. The second substance is megalin (gp330/LRP-2), an intracellular receptor which is expressed in embryonic epithelia and which has a role in growth {McCarthy, 2003}. The megalin receptor acts by linking Hh protein and retinol. Hh action is thus inhibited.
Unfortunately, a role of the sonic hedgehog pathway in SK is not proven and the two substances mentioned are not in the present acceptable therapeutic options. SK is not present in SK as it is in other malignant neoplasms: small cell lung carcinoma, medulloblastoma, basal cell carcinoma , suggesting it could be a mere benign hyperproliferative state.
Accumulation by senescence theory?
Ki67 has been measured in SK {Matsuta, 1996} by immunohistochemistry and the growth fraction (number of Ki 67 positive cells divided by the total amount of cells) amounted to 9.7% +/- 3,1%. The value was 3 times lower than in basal cell carcinoma (32.9 +/- 10,5%). Even if we take into account the lower growth fraction of ki-67 positive cells which is normally defined, there are laboratory variations. Moreover over studies{Gilhar, 2004 #429}that normal skin in old age is around 12 %. If we try to compare the two groups, SK appears to be hypoproliferating.
Nakamura and al think that SK is provoked by an accumulation of senescent keratinocytes expressing p16ink4a and unable to enter apoptosis. They(2003){Nakamura, 2003} found that all 10 tissue samples of acanthotic SKs expressed P16 (immunohistochemistry) in the whole epidermal thickness, p16ink4a being only present in the granular layer in the normal skin epithelium. Conversely, the granular layer is the layer of senescent cells in the normal epidermis. The following paragraph is summarised by the figure below.
CDKN2A is the gene which codes for protein 16 (p16) ink 4a, which is an inhibitor of a cyclin dependant kinase (CDK) {Castellano, 1997}. p16ink4a links CDK4 or 6, which are rendered unable to phosphorylate Rb, thus the cell cycle is blocked in the G1-S phase transition {Guan, 1994; Lukas, 1995; Koh, 1995}. This mechanism is probably responsible of senescence {Alcorta, 1996; Stein, 1999; Kiyono, 1998}.
Nakamura et al (2003) {Nakamura, 2003} also found an increased cell survival time, perhaps due to an inhibition of entry into apoptosis. No DNA fragmentation (TUNEL method) was found in 4 of 5 specimens. Thus keratinocyte cells cannot disappear as corneocytes. Indeed, p16ink4a in addition to stopping the cell cycle is known to inhibit apoptosis by the p19-p53 pathway {Quelle, 1995; Wu, 1994; Nevins, 1992; Qin, 1994} also found an increased cell survival time, perhaps due to an inhibition of entry into apoptosis. No DNA fragmentation (TUNEL method) was found in 4 of 5 specimens. Thus keratinocyte cells cannot disappear as corneocytes. Indeed, p16 in addition to stopping the cell cycle is known to inhibit apoptosis by the p19-p53 pathway {Quelle, 1995, Nakamura, 2003} also found an increased cell survival time, perhaps due to an inhibition of entry into apoptosis. No DNA fragmentation (TUNEL method) was found in 4 of 5 specimens. Thus keratinocyte cells cannot disappear as corneocytes. Indeed, p16ink4a in addition to stopping the cell cycle is known to inhibit apoptosis by the p19-p53 pathway {Quelle, 1995; Wu, 1994; Nevins, 1992; Qin, 1994}. No p53 was found, which according to Soini et al. {Soini, 1994} suggests that the SK samples were non proliferative.
Authors’ experience
We tried to see if SK was due to a hyperproliferation or an accumulation of cellular material.
Methods: We took after approval of the ethics committee 36 seborrheic keratosis and 15 specimens of control skin (plastic surgery, inflammatory skin (biopsies of suggested lymphomas with ki.67 analysis but with no epidermotropism or atypical cells) which were sent for histological analysis and ki-67 marking (method in detail). Two groups within the SK group were done: all histologies compatible with a reticulate SK or acanthotic SK were put in the RA (reticuloacanthotic group) and the hyperkeratotic SK were put in the AHCS group. There were no clonal specimens. We did not take into account the pigmentation of cells, nor their degree of irritation. The keratinocytes in SK and control skin was then done. All ki-67* fully positive cells and a fine estimate of the number of cells was done under a microscope lens of 40x magnification. An average of four adjacent fields was done. Then a ki-67 proportion index for control skin and SK was done by dividing the total number of cells.
*Only fully stained cells were counted, the other basal cells could have accumulated melanin which also appears brown.
“8” specimens of control skin and 5 specimens of seborrheic keratosis were stained by the TUNEL method. One specimen of control skin has irradiated by (ordi) UV B and then stained after 24 hours of immersion in a culture medium.
Results: The proportion of Ki-67 positive cells out of all SKS altogether equalled 2.3% +/- 0.3% whereas the one of control skin was 8.27% +/- 0.7%. One of the specimens was subtracted from the study because the proportion of ki-67 positive cells equalled 72%!There was no statistical difference between the two chosen histological types of SK( reticulo-acanthotic 2.0 +/- 0.5% versus acanthohyperkeratotic 2.6% +/- 0.3%. The difference was statistically significant between SK and the control group. The control group divided into two groups, one on inflamed skin and the other on normal skin showed a statistical difference with the inflammatory group showing a bigger percentage of ki-67 positive cells.
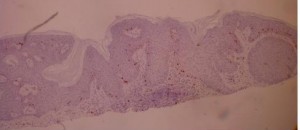
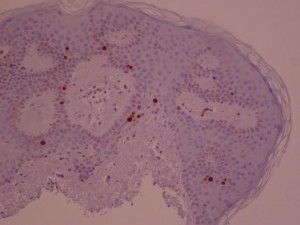 ki-67 staining of reticulo-acanthotic type of SKs.
ki-67 staining of reticulo-acanthotic type of SKs.
Ki-67 staining of an acantho-hyperkeratotic type of SK. On the last histological picture, most of the basal staining comes from the accumulation of pigment as the clinical image suggests.

Proportion of ki-67 positive cells. The values equal to 0% (All Acanthohyperkeratotic) were taken into account. Even if these values were not taken into account, the difference between normal skin and acanthohyperkeratotic and SK as a whole would not be significantly changed, as the standard deviation would be decreased.

Comparaison of reticulo-acanthotic subtype of SK, Acanthohyperkeratotic, these two histological subtypes combined and normal skin. The average normal proliferating skin has an average of ki-67 positive cells of 8.27 percent which is at least 250% of the SK ki-67 percentage of positive cells (2.3%) KStotale=all SKs studied KS A=acanthotic or reticulate SKs KS AHCS=acanthotic and hyperkeratotic SKs Controles=control group
Discussion: These results clearly show that the number of ki-67 in SK is either equal or lower than that of normal skin. The SK cells appear to be of a lower mitotic rate than control skin. Let us admit that we didn’t measure the basal layer proliferating index. But we can nevertheless say that if the number of cells increased because of a hyperproliferation, the number of ki-67 absolute number of cells would remain constant and that the proportion would remain unchanged.
There is no difference between the “proliferating” index between RA and AHCS SK. This means that although these lesions have clinically specific presentations (see our earlier experiment), there is no way of predicting the evolution of a lesion, a small and smooth SK can enlarge or remain static whereas an already large hyperkeratotic lesion can continue to grow.
The ki-67 marking of cells tends to be underestimated in our specimens. This is explained by a rigorous count of fully postitive ki-67 positive cells. The not fully stained cells resemble pigment accumulated in cells and thus the light accumulation of ki-67 positive cells were not counted. Thus the lower number of ki-67 postitive cells could be explained by laboratory methods or a lower amount of counted ki-67-positive cells( nomrmal values 9.7%+/- 3.1%{Matsuta, 1996}.
The difference in median age between the SK group and the control skin group could explain a bigger difference between SK in normal and control skin. According to {Gilhar, 2004}), group the ki-67 proliferating index is higher in a younger age group (%) than an older age group (12%). Even though the older age group shows a proliferating index of –%, laboratory differences make the comparaison between our group and theirs not comparable.
The TUNEL method of –SKs didn’t reveal any interDNA fragment staining in any of the SK and control skin specimens. The irradiated control skin (positive control) showed important DNA staining (photos à mettre)
Conclusion
As a conclusion two schools of thought exist, one advocating a retention of keratinocytes and the other a hyperproliferative state. A study in our department measuring the ki-67 index of “proliferation” of cells states that SK is the result of an accumulation of cells. The TUNEL experiment not showing any staining in the specimens of SK shows a supplementary argument to the presence of senescent cells, that is an inhibition of apoptosis.
One study relating bcl-2 expression is not incompatible with neither of the two schools of thought. Bcl-2 is a protein that inhibits apoptosis . Nakagawa et al (1994) {Nakagawa, 1994}evaluated bcl-2 expression in epidermal keratinocytic diseases. Immunohisochemical expression of bcl-2 in benign epithelial neoplasms (psoriasis, SK…) was compared with that of malignant skin tumours (squamous cell carcinoma, basal cell carcinoma…) Bcl-2 was totally absent in normal skin, scarcely present in SK, and present in all patients with squamous cell carcinoma. Malignant tumours thus have an enhanced cell survival. On the contrary, SK doesn’t behave like malignant neoplasms, and would be merely a bunch of keratinocytes in a hyperproliferative state.
As a conclusion inhibitors of apoptosis, whether survivin or bcl-2 are expressed in SK. Thus the retentionnal model is the plausible one our experience tends to support.
It would be interesting to measure by western blotting cell cycle proteins, ie. To repeat the Nakamura et al. of p16 expression. Another interesting path would be to measure SK keratinocytes’ resistance to apoptosis.
Pigmentation in SK: a mechanism explained by parallelism?
The mechanism in classical SK of pigmentation is unknown. It has nevertheless been studied in melanoacanthoma, a histologically close lesion to SK, which is sometimes considered as a subtype of SK, but is located in the muquous membranes. Pigmentation in SK is postulated to come from the activation of the endothelin receptor by endothelin-1 (ET-1) {Teraki, 1996}. ET-1, whose production is stimulated by UVB light {Kadono, 2001} is produced by endothelin converting enzyme 1 (ECE-1). Identification of ET-1 was done using immunohistochemistry and reverse transcriptase-polymerase chain reaction(RT-PCR) on seven acanthotic and pigmented SKs. RT-PCR revealed an increase in mRNA of ET-1 and tyrosinase, suggesting an ET-1 role in pigmentation. The postulted mechanism of action is as follows :.
Endothelin then acts on the neighbouring melanocytes (paracrine regulation) which express the endothelin receptor and induces melanocyte mitosis and production of melanin {Imokawa, 1992; Yada, 1991}.
Then the melanocytes transfer their melanosomes to the neighbouring keratinocytes {Sanderson, 1968}.
Category : growth mechanism - Modifie le 10.5.2012Category : Kératose séborrhéique - Modifie le 10.5.2012Category : mécanisme de croissance - Modifie le 10.5.2012Category : Seborrheic keratosis - Modifie le 10.5.2012Category : Seborrhoeic keratosis - Modifie le 10.5.2012Category : verrue séborrhéique - Modifie le 10.5.2012